| |
As peripheral artery disease (PAD) puts thousands of patients around the world at risk of mortality or amputation, it is still a disease that is comparatively underdiagnosed and undertreated. Currently, nearly 50% of PAD patients are unaware of the disease and have no symptoms to identify. Therefore, medical doctors and researchers are committed to developing a noninvasive and portable diagnostic system to obtain the result as quickly and accurately as possible.
|
|
|
| |
 The customer is a start-up company in the field of medical equipment manufacturing. It has developed a portable medical device that is designed to screen, stage, and monitor diabetic foot and PAD as early as possible to prevent amputation or ischemia in the worst-case scenario. To develop the most capable system, the customer turns to single board computer (SBC) to provide the system with computing, dashboard display, wireless connection, and rich I/O for sensors. The customer is a start-up company in the field of medical equipment manufacturing. It has developed a portable medical device that is designed to screen, stage, and monitor diabetic foot and PAD as early as possible to prevent amputation or ischemia in the worst-case scenario. To develop the most capable system, the customer turns to single board computer (SBC) to provide the system with computing, dashboard display, wireless connection, and rich I/O for sensors.
The customer was looking for a 3.5” embedded board to integrate into its portable PAD diagnosis system. This industrial-grade motherboard aims to process massive data such as blood flow, oxygen flow in the foot, the severity of ulcers or gangrene, and infection, and then display a dashboard on a monitoring screen to provide real-time information to the doctor. It is required to support the high-performance Intel® Core™ processor. Considering the CPU shortage, the customer specifically pointed out that the SBC should be in-stock and ready to ship.
|
| Main Requirements |
- Compact 3.5-inch embedded board
- Intel® Core™ processor
- Various display outputs choices
- Great expansion for more storage capacity
- Rich I/O connectivity
- Wide operating temperature range
- In-stock and ready to ship
|
| |
|
Axiomtek’s 3.5” embedded SBC CAPA500 provides capable performance
|
|
| |
|
 After a careful evaluation of CPU performance and the inventory, Axiomtek has proposed its CAPA500, a high-performance 3.5” embedded SBC. This industrial-grade SBC is powered by the 7th/6th gen Intel® Core™ i7/i5/i3 processor. It comes with triple view displays with one VGA, one HDMI, and one LVDS. Once it’s integrated with the system, which allows doctors to gain a clear view of the dashboard of various medical data; it’s equipped with three USB 3.0 ports and USB 2.0 ports for a variety of sensors. For more storage space or expansion, the CAPA500 features a full-sized PCI Express Mini Card slot with mSATA supported for data storage. The small form factor embedded platform also features two GbE LAN ports for the internet connection to transmit data and patient records; if there’s a need for a wireless connection, the system can gain a 4G/Wi-Fi connection via an additional module. The SBC has a ZIO connector for additional PCIe x1, LPC and USB ports. After a careful evaluation of CPU performance and the inventory, Axiomtek has proposed its CAPA500, a high-performance 3.5” embedded SBC. This industrial-grade SBC is powered by the 7th/6th gen Intel® Core™ i7/i5/i3 processor. It comes with triple view displays with one VGA, one HDMI, and one LVDS. Once it’s integrated with the system, which allows doctors to gain a clear view of the dashboard of various medical data; it’s equipped with three USB 3.0 ports and USB 2.0 ports for a variety of sensors. For more storage space or expansion, the CAPA500 features a full-sized PCI Express Mini Card slot with mSATA supported for data storage. The small form factor embedded platform also features two GbE LAN ports for the internet connection to transmit data and patient records; if there’s a need for a wireless connection, the system can gain a 4G/Wi-Fi connection via an additional module. The SBC has a ZIO connector for additional PCIe x1, LPC and USB ports.
|
| |
| System Configurations: CAPA500 |
- LGA1151 socket 7th/6th gen Intel® Core™ i7/i5/i3 processor (codename: Kaby Lake/Skylake)
- Intel® H110 chipset (Q170 optional)
- 1 DDR4 SO-DIMM for up to 16GB of memory
- 3 USB 2.0 and 3 USB 3.0
- Intel® AMT 11 supported (optional)
- Dual-view for H110 chipset; triple-view for Q170 chipset
|
| |
|
Application: The CAPA500 makes the medical examination safe and easy
|
|
| |
|
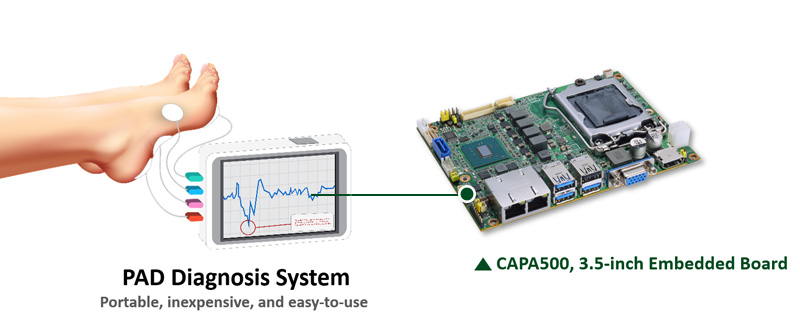 The customer has integrated the CAPA500 into its PAD diagnosis system for subdermal noninvasive monitoring. With the support of the PAD diagnosis system, the doctor can easily screen the patient’s feet and evaluate the severity of the disease and make decisions on treatment. The device can show the precise location and severity of the disease and can even be used during surgery. The examination is safe, and the system is relatively inexpensive and can transmit data via the cloud. The customer has integrated the CAPA500 into its PAD diagnosis system for subdermal noninvasive monitoring. With the support of the PAD diagnosis system, the doctor can easily screen the patient’s feet and evaluate the severity of the disease and make decisions on treatment. The device can show the precise location and severity of the disease and can even be used during surgery. The examination is safe, and the system is relatively inexpensive and can transmit data via the cloud.
|
| |
|
|
| |
|
Axiomtek’s products meet the demand of the customer and become an essential part of the subdermal noninvasive monitoring system. Now the development of the system has been completed and received multiple patents. The examination is non-invasive and ionizing-radiation-free. Moreover, it is expected to be certified by the local authority.
“After we have approached Axiomtek from the website ads, its local partner sent us a display kit in a very short time. Since the short supply of chips remains in 2022, having a quick-respond service and ready stock to market is the main reason we choose to work with Axiomtek. It offered a quick and proper option – CAPA500 – with 7th Intel® Core™ i5 and i3 processors to meet different needs in hospitals. Axiomtek shows its powerful coordinated ability and shrewd strategy in this competitive time,” said the Chief Technology Officer of the PAD Diagnosis System Provider.
|
| |
|
|